Abstract
Introduction: CXCR4 (CD184) is a G protein-coupled chemokine receptor specific for CXCL12 that is associated with leukocyte chemotaxis. It is expressed on normal hematopoietic cells as well as hematopoietic and epithelial cancer cells. CXCR4 and CXCL12 have been associated with bone marrow retention of AML and ALL blasts and blocking this interaction via small molecule inhibitors has been investigated as a therapeutic target. CXCR4 has also been implicated in other aspects of tumor progression including metastasis, angiogenesis, and survival. As part of Children's Oncology Group protocol AAML1031 for de novo pediatric AML we prospectively evaluated CXCR4 expression by difference from normal (ΔN) flow cytometry and correlated expression with clinical characteristics and outcome. Given the impact of the bone marrow niche on the administration of chemotherapy we hypothesized that high levels of CXCR4 expression would be associated with unfavorable outcomes.
Methods: In the AAML1031 phase 3 trial pediatric patients with de novo AML were randomized to receive standard chemotherapy (Arm A) or standard chemotherapy with bortezomib (Arm B), excepting patients with high allelic ratio FLT3-ITD who received standard chemotherapy plus sorafenib (Arm C). Patients were stratified as low-risk (LR) or high-risk (HR) based on cytogenetic and molecular abnormalities, and end of induction (EOI) minimal residual disease (MRD) by flow cytometry. LR patients received four cycles of the standard chemotherapy backbone while HR patients received three followed by best hematopoietic stem cell transplant. Patients who: (1) submitted a bone marrow aspirate for ΔN at diagnosis and (2) provided consent for correlative biology studies were included in this analysis. All diagnostic specimens were centrally and prospectively evaluated for the expression of CXCR4 by ΔN.
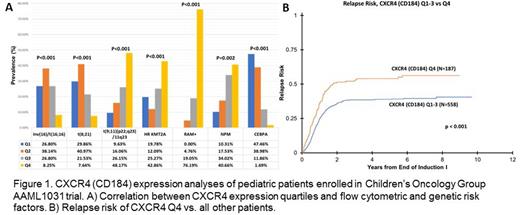
Results: Surface CXCR4 expression was available for 1004 patients and expression on AML cells varied across the cohort (range: 54-17,793 molecules/cell). For analysis, the study population was divided into quartiles (n=257-258 patients each) based on expression levels. Quartiles showed no association with sex and were distributed as expected between treatment arms. Correlation with clinical characteristics showed that patients in the top quartile of CXCR4 expression were younger (5.9 vs. 11.4 years, P<0.001) and had higher bone marrow (BM) blast counts but lower peripheral blood (PB) blast counts (76% vs. 66%, p<0.001 and 33% vs. 43%, p=0.01, respectively). These patients were highly enriched for HR KMT2A fusions (43% of total fusion positive patients), as well as t(9;11) and NPM1 mutations; while they had a lower frequency of t(8;21), inv(16), and CEBPA mutations (p<0.002 for all). There was no association between CXCR4 expression and protocol risk group assignment or complete remission rates, but patients in the high expressing CXCR4 quartile were more commonly MRD negative at EOI (p=0.03). Flow cytometry also revealed a striking increase in RAM phenotype (p<0.001), with 76% of all RAM patients allocated to the CXCR4 high expressing quartile.
Patients in the top quartile of CXCR4 expression had lower 5-year OS (58% vs. 66%, p=0.036) and EFS (38% vs. 47%, p=0.009) compared to other patients. CXCR4 was strongly associated with increased 5-year relapse risk (RR) (54% vs. 39%, p<0.001). Among patients with HR KMT2A fusions high expression of CXCR4 was associated with an increased 5-year RR (85% vs. 57%, p=0.04). Multivariable analysis for 5-year RR including MRD status and prognostic genetic factors indicates CXCR4 expression is independently associated with relapse, with a hazard ratio of 1.33 (95% CI 1.04-1.7; p=0.026).
Conclusions: These data demonstrate that CXCR4 expression is associated with individual HR markers (RAM phenotype and HR KMT2A fusions), but not risk status across the entire study. Despite similar risk status, patients in the top quartile of CXCR4 expression had inferior outcomes. These patients had higher BM blast counts but lower PB blast counts, which has been observed in RAM phenotype, likely due to the impact of CXCR4 on bone marrow retention of leukemia cells. This increased retention may also play a role in treatment resistance, suggesting that the addition of small molecule inhibitors of CXCR4/CXCL12 to chemotherapy may help these high expressing patients, especially in subtypes such as HR KMT2A and RAM phenotype patients.
Disclosures
Menssen:Hematologics Inc.: Current Employment. Hudson:Hematologics, Inc.: Current Employment. Pardo:Hematologics Inc.: Current Employment. Hsu:Hematologics Inc.: Current Employment. Lott:Hematologics Inc.: Current Employment. Dai:Hematologics Inc.: Current Employment. Ghiradelli:Hematologics Inc.: Current Employment. Loken:Hematologics Inc.: Current Employment, Current equity holder in private company. Eidenschink Brodersen:Hematologics Inc.: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal